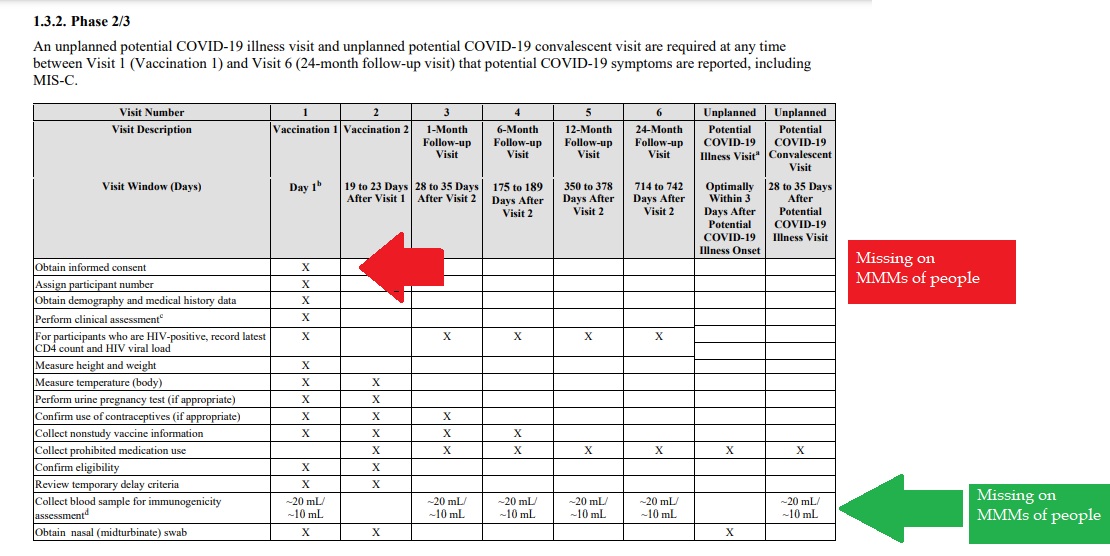
The 1974 Research Act was created in entirety from the Belmont report, and put into place to prevent the Government, it agencies or representatives, military and private companies, from violating an individual's freedom: by forcing, tricking or coercing persons for research, testing and administration of unknown injections/materials, and experimental procedures. This law was enacted after a century long track record of precedence including, and not limited to, the following:
Demonstrated lack of Respect for Persons and their protections in violation of 1974 law. 1- Autonomous agents, individuals capable of deliberation about personal goals and of acting under the direction of such deliberation. The Government and companies have to give weight to autonomous persons' considered opinions and choices, and cannot obstruct their actions and judgments, nor deny individual freedom to act on considered judgments, and cannot withhold information necessary to make a considered judgment. Necessary information includes - but not limited to - all medical opinions by established, industry experts, health status, the necessary assessments, labs, with close monitoring of physical lab and test follow ups of each and every autonomous persons as part of clinical research and testing, along with full documentation of testing, efficacy, use of chimera for research and testing, any and all conceivable side effects, and interactions of conditions. 2- persons with diminished autonomy are entitled to added protections. Violators to the law have been marketing to the most vulnerable, including the immature and the incapacitated who were in need of extra, added protections, even to the point of excluding them from any injections or procedures which may harm them; violating added safety precautions for children, elderly, or those with diminished capacity. Informed consent - must include full disclosure of ALL contents to be injected, any and ALL possible side effects (which can be several pages long), how those persons are individually to be closely monitored, safety guidelines, and above all the right to say no before or at any time, and full reporting of all individuals regarding their safety monitoring/labs/assessments, and any and all side effects. By promoting COVID shots all still under clinical trial/research, and coercing with careers/jobs, inability to travel, etc. in order to take the shots, this law is being violated through: lack of informed consent, lack of protections of autonomous persons, and/or illegally acting as IRB safety board members marketing to those not autonomous and capable of self-determination, with higher standards of protection to be invoked, and assuming the role for their safety. The maxim "do no harm" has long been a fundamental principle of medical ethics. Claude Bernard extended it to the realm of research, saying that one should not injure one person regardless of the benefits that might come to others. An agreement to participate in research constitutes a valid consent only if voluntarily given. This element of informed consent requires conditions free of coercion and undue influence. Undue influence also includes offers of an excessive, unwarranted, inappropriate or improper reward or other overture in order to obtain compliance. Also, inducements that would ordinarily be acceptable may become undue influences if the subject is especially vulnerable as in the case of targeting children, persons with limited capacity, and elderly with elements of mental defect, or instilling fear. Short term morbidity and mortality cases from the shots are well reported and known, such as death, myocarditis along with spontaneous cardiac arrest with no warning, debilitating neurological conditions, etc. And, there is no means yet to determine mid and long term effects because Phase I trials have not been competed, let alone Phase II and III - which is vital information in order to determine informed consent. Injustice has been performed with companies and government representatives, by involving vulnerable subjects, including the young, those unable to fully comprehend with all necessary information, and scaring parents with compromised capacity for free consent. In addition to lack of individual, tightly scheduled, continual monitoring and follow ups, autopsies of all persons involved in this trial participation should be conducted for reporting by the pharmaceuticals companies for any and all persons who received the shots, as well as labs determining efficacy and detriments (as examples, antigen creation, D-dimer, Pulse Cardiac and Troponin Tests) for all those who were coerced or unduly influenced to participate in research. This law was created to protect people from government abuse through experimentation. The government cannot arbitrarily dismiss components, create resolutions or stipulations to supersede the law, as to invalidate its protection of individuals from them, including, but not limited to Health and Human Services (HHS) Center for Disease Control (CDC), Food and Drug Administration (FDA), National Institute for Health (NIH), etc. and pharmaceutical companies, etc. Persons have been illegally acting as members of, or bypassing, IRB safety review and monitoring of each and every person receiving injections, with open undue influence and coercion, to participate in Covid injections. Coercion has been especially directed to the diminished autonomous, children and elderly, through TV ads, library recordings, verbal encouragement, schools or other public venues acting as government agents, and/or clinical researcher recruiting participants, and/or illegally as untrained IRB member who is not following up to ensure safety of the people they recruited, coerced or used undue influence. Overall lack of informed consent has become too often common practice across the healthcare industry, including people being given consent forms hours or minutes prior to surgery; no alternative treatments or lifestyle-nutrition changes prior to medications begin prescribed, and undue influence to intubate or perform surgery on patients in lieu of alternative treatments.
Lawsuits for unconstitutional violation of laws regarding shotsPosted by Brian Ward on Twitter Guess what? Defendants in our lawsuits no longer argue that they had a right to mandate EUA drugs due to their state's at-will employment doctrine. That the EUA drugs can be mandated. That they had the authority to even issue the mandate. Why? Read the 127 pages, and then you'll understand that it was a legal lie from the beginning, but due to the novelty of the laws, no one knew of them. As courts have stated, "sometimes laws take naps," and these laws never showed up for work until now. https://coloradomedicalfreedom.com/wp-content/uploads/2023/08/Stamped-Final-Complaint.pdf full document https://coloradomedicalfreedom.com/wp-content/uploads/2023/08/Stamped-Final-Complaint.pdf page 127 C. the Secretary has no “authority to require any person to carry out any activity that becomes lawful pursuant to an authorization under this section…” 549. In 2005 Congress passed the PREP Act94 which provided the following regarding preemption of state law: (8) During the effective period of a declaration under subsection (b)…no State or political subdivision of a State may establish, enforce, or continue in effect with respect to a covered countermeasure any provision of law or legal requirement that— (A) is different from, or is in conflict with, any requirement applicable under this section; and (B) relates to the…administration…of the covered countermeasure, or to any matter included in a requirement applicable to the covered countermeasure under this section or any other provision of this chapter, or under the Federal Food, Drug, and Cosmetic Act [21 U.S.C. 301 et seq.]. 550. Therefore, via the PREP Act and 21 U.S.C. §360bbb-3, Congress expressly prohibits Defendants from: A. interfering with the authority of the Secretary, B. establishing a condition not authorized by the Secretary, C. establish conditions contrary to the Secretary and the congressional statute under 21 U.S.C. §360bbb-3, D. mandate participation in any 21 U.S.C. §360bbb-3 product or PREP Act activity, E. interfere with an individual considering participation in a 21 U.S.C. §360bbb-3 product or PREP Act activity, F. penalize a person refusing to participate in a PREP Act product or activity or 21 U.S.C. §360bbb-3 product. 551. The executive branch of the United States Government purchased all COVID-19 licensed and EUA drugs using federal funds. Congress expressly prohibits the federal government Brian Ward @GodsRiddles Nov 1, 2023 Breaking…Gov Gavin Newsom and Kaiser have been sued in federal court for requiring healthcare workers to inject an experimental drug into their bodies as a condition to sell their labors in the marketplace. The requirement violated the workers’ Equal Protection of Laws and Due Process rights. Moreover, Kaiser signed a contract with the CDC promising not to mandate participation but did so anyway. Kaiser and Newsom fraudulently concealed the fact that nurses would be required to forfeit litigation rights if they incurred an injury from the use of the experimental drug. The California Nurses union stood by and refused to stop Newsom’s tyranny, leaving members without representation. Governor Newsom perpetuated the greatest assault on the US Constitution in the state’s history and the CA AG did nothing to prevent it. Worse yet, is that CA and Kaiser already had an agreement with HHS promising to never place an individual under a sanction for refusing to inject federally funded experimental drugs into the body. Legal Fact: medical providers have dual roles in relation to their employees. An employee can be a patient and an employee. Should a hospital mandate the use of a drug under the PREP Act as a condition of employment and the patient is injured then the patient would find it difficult to sue the medical provider for that injury. However, the employee has the right to seek compensation irrespective of the PREP Act because it’s an on the job injury. Of course this opinion does not account for the laws of all 50 states but demonstrates why one should seek legal advice immediately upon sustaining an injury. This is not my opinion but the chief judge of the 11th circuit who wrote a slip opinion when he was AL AG denoting the company is liable for vaccine injuries because the company believes the vaccine would benefit the company. Last edited10:55 AM · Nov 10, 2023
Brian Ward - Twitter Oct 25, 2023 Legal Fact: Any state that penalized citizens or denied unemployment benefits to an individual terminated for the sole reason of refusing to inject an unlicensed investigational drug into their body violated the individual's Fourteenth Amendment Equal Protection and Due Process rights. The U.S. Congress conferred legal authority onto an individual to either accept or refuse an EUA product. Both options were enacted by a valid act of Congress and must be equally protected. Moreover, the Supremacy Clause of the Constitution denied that state authority from interfering in the chosen option. The state established what courts call an Unconstitutional Condition. SCOTUS said: "But the power of the state in that respect is not unlimited; and one of the limitations is that it may not impose conditions which require the relinquishment of constitutional rights. If the state may compel the surrender of one constitutional right as a condition of its favor, it may, in like manner, compel a surrender of all. It is inconceivable that guaranties embedded in the Constitution of the United States may thus be manipulated out of existence." Governors and state agency directors violated their oath of office & the Constitution the moment they required COVID-19 EUA participation and or penalized an individual's federally protected option. They deprived citizens of their interest in liberty and property and, unfortunately for some, their very lives, all without due process. They did so by applying the law unequally demoting those who refused to that of a second-class citizen. Legal Fact: 21 U.S.C. §360bbb-3 and the PREP Act expressly restrict public and private employers from conditioning access to employment and other benefits upon a person injecting an FDA-classified experimental drug (Pfizer-BioNTech COVID-19 Vaccine) into their body. The CDC stated, "Coverage under the Public Readiness and Emergency Preparedness (PREP) Act extends to Organization if it complies with the PREP Act and the PREP Act Declaration of the Secretary of Health and Human Services." "IF IT COMPLIES" is the key phrase here. When a governor issued a proclamation that directly violated the federal statute, the governor did not comply and fraudulently amended the federal law in violation of the Supremacy Clause. The PREP Act and 21 U.S.C. §360bbb-3 provide a legal right to individuals considering participation in the product/activity. That legal right is the option to accept or refuse. Therefore, the immunities provided to persons participating in activities under the PREP Act extend only so far as they do not force persons to participate in the product/activity. Governors, employers, hospitals, all FORCED (under threat of a penalty) individuals to participate in violation of federal law. Force does not have to mean physical force. It can be the force of law, rule, or other means having the same negative effect as physical force. The courts have said that force means to prevent a person or cause a person to participate in an activity outside of their free will and voluntary consent. Significant lawsuits inbound! 12:45 PM · Aug 29, 2023 Brian Ward Aug 2022 BREAKING: LA Superior Court requiring LAPD to reinstate an officer terminated for refusing the vax mandate. Full back pay too! This is under a Writ of Mandamus, which is rare. Legal Fact: "Nothing in this section (EUA Law) provides the [HHS ] Secretary any authority to require any person to carry out any activity that becomes lawful pursuant to an authorization under this section, and no person is required to inform the Secretary that the person will not be carrying out such activity." - The Secretary may grant access to an unlicensed drug (Pfizer-BioNTech COVID-19 Vaccine) during an emergency but he can not mandate that anyone manufacture, distribute, store, administer, or receive the product. His authority is non-transferable, nor may he delegate it to another person. Therefore, by what authority are universities and private employers mandating that which Congress prohibits? EUA drugs are "controlled" drugs by Congress, and no person may participate in them outside of the conditions established by Congress. Moreover, Congress expressly prohibits private employers from interfering with your choice of accepting or refusing participation in the product. It was illegal - It is illegal - and it is being remedied in court. We will not allow this September to be a repeat of years past. 2:18 PM · Aug 21, 2023 Legal Fact: 100% of all hospitals and nearly all universities signed a FEDERAL agreement to abide by the ethical principles of the Belmont Report, though few know it, ANYTIME they involve a human with an investigational medical product such as any available COVID-19 drug. The report is only 10 pages long, but it holds in part: (1) Respect for persons incorporates at least two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection. The principle of respect for persons thus divides into two separate moral requirements: the requirement to acknowledge autonomy and the requirement to protect those with diminished autonomy, (2) To show lack of respect for an autonomous agent is to repudiate that person's considered judgments, to deny an individual the freedom to act on those considered judgments, or to withhold information necessary to make a considered judgment, (3) Respect for persons requires that subjects, to the degree that they are capable, be given the opportunity to choose what shall or shall not happen to them. BONUS ROUND - All US States and Territories signed this agreement pre-pandemic too. Yup, no one knows this either, including state attorneys general. Lastly, unlike the Nuremberg Code, the Belmont Report has the force of law via federal statute and contract. Last edited9:28 PM · Aug 2, 2023 Brian Ward via Twitter
The VERY first EUA issued was in 2005 for the Anthrax investigational drug for service members and civilian employees of the DoD. The EUA stated: A. Individuals (service members and civilians) who refuse anthrax vaccination will not be punished. (Emphasis added) B. Refusal may not be grounds for any disciplinary action under the Uniform Code of Military Justice. C. Refusal may not be grounds for any adverse personnel action. Nor would either military or civilian personnel be considered non-deployable or processed for separation based on refusal of anthrax vaccination. D. There may be no penalty or loss of entitlement for refusing anthrax vaccination, E. This information shall read in the trifold brochure provided to potential vaccine recipients as follows: You may refuse anthrax vaccination under the EUA, and you will not be punished. No disciplinary action or adverse personnel action will be taken. You will not be processed for separation, and you will still be deployable. There will be no penalty or loss of entitlement for refusing anthrax vaccination. Nothing in law has changed to negate the authority of DoD members to refuse EUA COVID-19 drugs except the 6 civilian appointees engaged in willful misconduct against our Armed Forces. 11:51 PM · Sep 3, 2023
1 Comment
What should you be aware of regarding Vancomycin?by Rose Rohloff
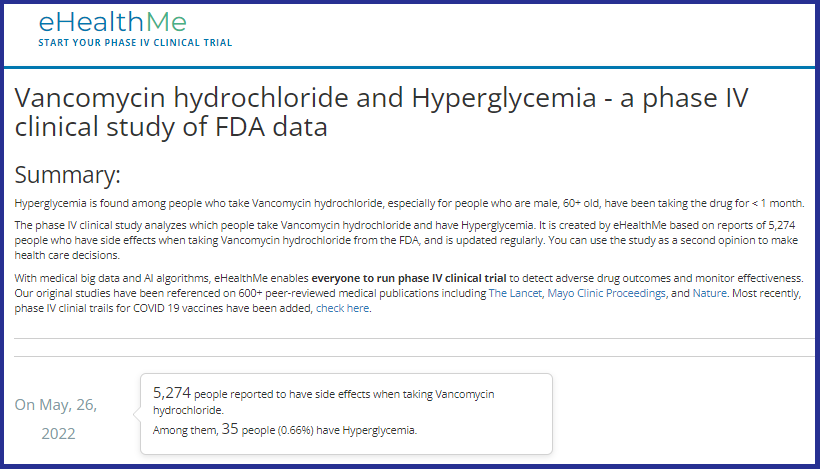
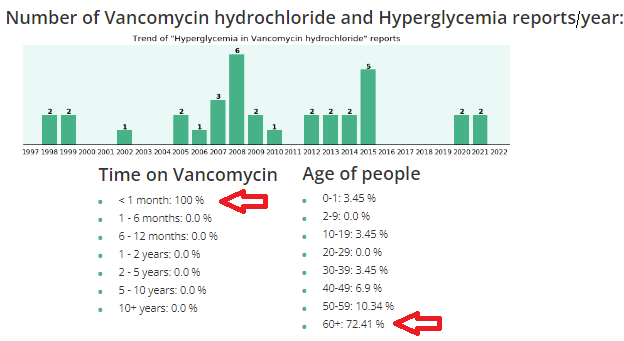
proliferation of the intestines (often after antibiotic use); and some hospitals are using as the standard protocol for elderly in the emergency room, for asymptomatic pneumonia, and other infections. Clinicians (nurses, doctors, physician assistants, etc.) are supposed to do comprehensive history and physicals (H&Ps) before using any drugs or treatments. And, it is important to know underlying conditions before using Vanco, because it can raise glucose levels, especially in diabetics, and/or cause kidney (renal) insufficiency, especially in elderly. The following use case is regarding an admitted primary care doctor as the patient. "I don't know what the average "lay person" does when they don't have all this information, and without a medical person to look out for them." "This article is exactly why they stopped Vanco when he had his MRSA infection. His kidneys were starting to get compromised, so they immediately stopped it and started him on a different antibiotic, Daptomycin, which worked just as well and had less side effects for his kidneys and diabetes. Thankfully they caught it very early because of the blood tests they were doing to see what was happening. The new antibiotic was infused only once a day as opposed to twice a day Vanco, and there weren't all the extra blood draws to make sure the kidneys and glucose were doing okay. God is really in control and watching out for us, because the medical field doesn't always do that, even with a fellow medical person." Vanco and high glucose/hyperglycemiaVanco and kidney (renal) failureChanges in vancomycin use in renal failure Stefaan J Vandecasteele 1, An S De Vriese pub in 2010 Abstract A progressive increase in vancomycin resistance with consequent treatment failure has been observed in staphylococci. Therefore, new dosing guidelines advocating much higher vancomycin doses have been issued. Target trough levels of 15-20 microg/ml are proposed. Whether and how these targets can be achieved in patients with chronic kidney disease or those on dialysis are still under evaluation. The higher vancomycin doses to achieve these treatment targets carry a substantial risk for nephrotoxicity. This risk is incremental with higher trough levels and longer duration of vancomycin use. Critically ill patients, patients receiving concomitant nephrotoxic agents, and patients with already compromised renal function are particularly at risk for vancomycin-induced nephrotoxicity. Acute kidney injury during daptomycin versus vancomycin treatment in cardiovascular critically illConclusions: Daptomycin appears to be safer than vancomycin in terms of AKI risk in ICU patients treated for cardiovascular procedure-related infection. Daptomycin could be considered as a first line treatment to prevent AKI in high-risk patients. NIH 2019
by Linda LybertPresident/ Healthcare Surfaces Expert, Founder/Executive Director Healthcare Surfaces Institute Privacy curtains must be addressed and as I talk with healthcare professionals about this issue I get mixed reactions. Digging deep into the issue the biggest problem is the amount of time and labor it takes to actually change the curtains out. "It is backbreaking and we don't have enough staff to actually do this on a regular basis." Every facility sets its own standards for changing privacy curtains and the responsibility lays with operations. Policies range from change when visibly soiled (NOTE: microbes can't be seen) to once a quarter and even once a year and any time in-between. As research continues to be published it is clear to mitigate the spread of infections solutions this must be part of a solutions bundle addressing all surfaces! See LinkedIn Post with full Study: Patient Privacy Curtains represent Infection Risk Linda Lybert is an amazing expert in healthcare surfaces. The same circumstances existed 20 yrs ago, but clinicians were properly trained & executed safety processes/avoided cross contamination; learning in school movement memory e.g. wash hands after closing curtains before touching patients, use elbows to open curtains to enter or push all the way open, etc. #1 issue - lack of clinical training, laziness, and inadequate adherence of movement memory for proper safety protocols. Rose Rohloff
by Rose Rohloff The 2009 HITECH Act and the Center for Medicare Medicaid Services’ (CMS) Meaningful Use regulations caused a massive spend for electronic medical records (EMRs), the push for interoperability, as the solution to healthcare quality. However, EMRs are not solutions - along with massive IT overhead spend with decreasing quality - because in a high percent of instances, nurses and doctors don’t even read them. A 40-year old mother went to the doctor after treating herself holistically for some laryngitis, stuffy nose, congested sinus, with continued symptoms after five days. After an exam, the doctor stated, “I am not going to give you antibiotics. You do not have a fever; your lungs sound clear. It looks like a little virus with severe allergies. I recommend an antihistamine.” The patient told him, “Thank you for not putting me on antibiotics when they are not needed, that makes me happy.” He responded, “I am glad you are glad.” He then said something and the patient responded, “I have MS.” He responded, “Oh wait, you have Multiple Sclerosis?”
This story is sadly too often the new normal, numerous instances of patients and their caregivers stating issues of diagnosing with medication prescription, or misdiagnosis; the doctor or nurse having no idea of pre-existing conditions or a full list of medications currently being taken, a lack of care coordination or care planning because the time was not taken to simply read the chart (whether written or electronic), and ensuring a comprehensive history followed by the necessary physical assessment. No physician or nurse should walk in to care for a patient without first having read the patient’s record, knowing all current information, the last visit/healthcare encounter, chronic conditions/comorbidities, and all medications; then, asking for updates of changes. Unfortunately, even without having to decipher poor handwriting, being able to read clean typed text, clinicians are not simply reading the basics of information they should before doing any diagnosis, planning and care, or prescribing of medications.  by Rose Rohloff A middle aged male was recently experiencing severe abdominal pain, subsequently prescribed three (3) medications in two (2) weeks from three (3) different sources (an Emergency Room, a primary care doctor, a Gastroenterologist). There was no diagnosis, no care coordination within an established plan of care, no thorough instruction in the medications, with the last prescription based on a guessed misdiagnosis which worsened his pain. One prescription was a steroid with the patient being instructed to take as he needed it; the second was an offering by the office secretary blindly asking if he wanted an Epipen when he called to actually speak with the physician for worsening abdominal pain, swelling and to discuss his lab work. The common standard operating procedure (SOP) in medicine has become symptom and write a prescription, another symptom and write another prescription, etc. This SOP has lent to the opioid crisis, antibiotic resistance, as well as many other drugs being dispensed routinely with side effects causing secondary prescriptions for the side effects of the existing medications being taken. Several variables cause the use of this SOP beginning with the lack to get a full, detailed history - taking time to speak with patients - to establish a diagnosis and then plan of care, determining if simple steps are first needed such as icing and therapy for pain before opioids, or to remove foods and medications isolating side effects or allergies. Last week, I attended the HIMSS conference, the largest healthcare conference in the country, with attendees from around the world. One executive stated, "I just returned from Finland where they have an effective health system, because people live healthy, and the doctors appropriately tell their patients NO when seeking a simple, quick fix of a drug that is not needed." Reasons for the mainstream SOP? I think there are always multiple reasons for issues within healthcare. The symptom=prescription issue can be: Doctors are processing patients through with 'factory-care', Physicians receiving kickbacks from pharmaceutical companies; The lack of proper clinical training; Protocols blindly being followed without individual evaluation (e.g. Vanderbilt University study on Plavix standard for all Cardiac Cath Patients); as well as the alliance of public policy and pharma, direct consumer marketing without proper education. A healthcare executive summarized the situation well last week when stating to me, "I ultimately make the decision for my own care, with the advice of the physician. It is the doctor's role to diagnosis, and then we discuss all options, along with a plan of care, coordinated with speaking with all other involved physicians." It is important for consumers to understand the need to champion their own care working with physicians, determining what options should be used before medications (diet and some of the old fashioned home remedies still hold true), addressing underlying issues versus only symptoms, and removing or changing medications to eliminate side effects when there are alternatives. Questions to have answered: An example of direct consumer marketing lacking in education: In 2016, there was broad publication when the company Mylan raised the prices of the Epipen after State Law was passed to stock it in every school. Many individuals and groups were upset because there is not a generic offering. With proper information, the public would be educated that Epipen is the patented delivery system, not the drug epinephrine. The generic already existed in the form of a $15-$18 sterile needle. It is also necessary to establish where and when is it appropriate to stock epinephrine, not specifically the Epipen. Why are you prescribing this medication, what is it specifically doing in my system? What are non-medication alternatives, what are other medication alternatives? How long should I take this, what is the outcome? How does it interact with my other medications? What should be monitored for an outcome, side effects? by Rose Rohloff
“We think sometimes we’re only drawn to the good, but we’re actually drawn to the authentic. We like people who are real more than those who hide their true selves under layers of artificial niceties.“
Elisabeth Kübler-Ross, Passion for Patients, (page 62) by Rose Rohloff The greatest surface for cleanliness is the hands of all personnel within any healthcare providing environment. There have been arguments with the increase of hospital acquired infections (HAIs) that there needs to be expensive initiatives for reminder programs to wash hands, or to institute check list programs for clinicians to stop and go through a clean protocol before performing care. However, there is a flaw and unnecessary high expense to this approach. Recently, the April 2007 story of Chief Mike Day, Navy SEAL, has been recirculated. The incident involved Day being shot point blank, 27 times (11 in his vest and 16 times into his body), within a 12 x 12-foot room, the gun fight occurring within seconds at a range of ten feet. After his rifle was shot out of his hands, he grabbed his pistol, remaining in the fight, taking out the four insurgents, and then becoming stunned being hit by a grenade fragment. Upon regaining awareness, he immediately inquired if the room was clear, and then walked himself to the evacuation helicopter. In one of his interviews, he stated, “I just went to work, it was muscle memory, I just did what I was trained to do.” “… into a gun fight, I feel more comfortable in that situation, I feel more comfortable, I don’t think, I don’t have to think in that situation, I just react.”
Day’s statements exemplify an important component that has been lost in healthcare training - that of muscle, or specifically, movement memory. Clinicians are supposed to be trained in school regarding the need and proper technique for handwashing. More importantly, clinicians used to have extensive clinical time working in patient areas developing the movement memory for proper hand washing, and automatically keeping in mind what is clean vs. dirty, where established sterile fields are located with maintaining of sterile gloved hands. The training was extensive and repetitive, for clinicians to automatically move appropriately in fast paced, life threatening situations - to not have to think and just act. One common, simple example is the insertion of IVs for fluid administration or needles for drawing blood. The needle or IV cannula (the needle with covered sheath inserted into the vein) is sterile, with clinicians wearing nonsterile gloves. The skin is typically wiped with alcohol to clean, and then all too often clinicians press nonsterile gloved fingers on the cleaned skin to feel for the vein; thus, contaminating the cleaned surface of the patient’s skin where insertion directly into their vein will occur. Even though the nurse/doctor is wearing clean gloves, they are not sterile, and worn to protect the clinician. With repetitive movement training, clinicians would press to find the vein before properly cleaning the skin, and clean their gloved fingers at the same time as the patient’s skin. Two frequent complaints often heard from patients, "They dug around in my arm and could not find the vein, it was so painful." "They poked me five times because they did not know what they were doing." Blood draws and starting IVs is a skill, just like shooting at a target or in high stress a gun fight, that requires proper training of technique, and more importantly, repetitive practice - especially with the understanding when someone's life depends upon it. Additionally, the conditioned good technique should be second nature to purge ALL air from needles and tubing, including from the side ports of IV tubing, to prevent the potentially fatal embolus as a hospital acquired condition (HAC). With the great reduction of hands on clinical time in schools (with replacement of online theory, population/global health, writing, and shadowing nurses), this movement memory training has been lost, with the shift of cost to hospitals for training, buying expensive monitoring equipment, or addressing the subsequent HAIs/HACs. Bringing the ingrained, repetitive movement training back to school training would instill within clinicians and CNA/PCT caregivers the instinctual, reactionary awareness of dirty versus clean or sterile, and proper IV/needle insertion, while delivering care; whether normal daily care or imminent life versus death situations – because they just do what they are trained to do without having to stop and think through quality actions. by Rose Rohloff
Upon reading the article "Must have bachelor's degree: Hospitals' new requirement for nurses" concerning a report published by The Wall Street Journal, I wanted to provide perspective from experts with first-hand experience in the industry addressing points within and not included in the Wall Street Journal report. [read more] Published Becker's Hospital Review
“I spoke to more than 40 people for the story and heard many of the points you raise, unfortunately I could not include every nuance in a 700-word story. All the best, Anna” WSJ journalist |
click an articleto read and post comments Search topicselect category
All
search by date
July 2024
|
||||||||||||||||||||||||||||