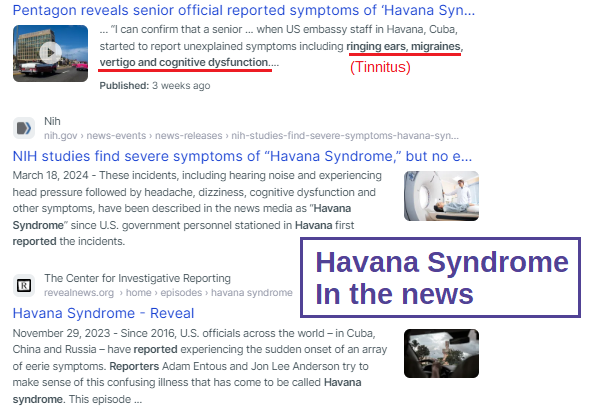
Who is in charge of healthcare? Is your Health insurance coverage invalidated/exempt? 5G and edc4/12/2024 by Rose RohloffEach individual should be in charge of their own healthcare decisions, safety surroundings, and be treated individually. So, a good question to ask, "Who actually is in charge?" Laws and Executive Orders (EOs) regarding healthcare under "public health"Created by Todd Calendar, Esquire H.R.3832 - Disease X Act of 2023 118th Congress (2023-2024) Open "disease" cart blanc for them to choose (or orchestrate?) ‘World Health Organization (WHO) Has No Authority to Dictate U.S. Health Policy’ factcheck.org March 2, 2023 "Although the accord is being called ... a treaty or another kind of binding agreement, such as the WHO Framework Convention on Tobacco Control, or a nonbinding agreement, such as the Paris Climate Accord. “As with all international instruments, any accord, if and when agreed, would be determined by governments themselves, who would take any action while considering their own national laws and regulations,” a WHO spokesperson told us." Federal and Local Governments would be in charge regarding any Rights violations. The accord is accumulation of monies $$$ to be "distributed" to various countries/persons, in the name of public health.
LTC (ret) Green Beret, Doc Pete Chambers, MD, bioweapons expertRisk of 5G rolled out, without safety studies, by Federal, State and Local governance. Communities were not asked regarding rollout, or informed regarding safety issues of 5G. Are we experiencing history repeating the dangers of DDT, Asbestos, many FDA "approved" drugs recalled because they lacked safety, etc.?
Are individual healthcare insurance policies, the underwriters of policies, being invalidated without the knowledge of people, and against their choice - through lack of knowledge, lack of informed consent, and through illegal coercion? Electromagnetic RadiationEndocrine Disrupting Chemicals (EDCs)
0 Comments
by Numerous expertsMy desire is to provide several articles from those adroit in the subject. COPPER: the less talked about, and important trace mineral, the importance of Zinc, and when taking high Zinc, make sure you know the ratio of zinc to copper supplements.
Ralph Baric and other scientists knew in 2010 the "antidote"/treatments for the upcoming virus(es). He coauthored the discovery "Increasing the intracellular Zn(2+) concentration with zinc-ionophores like pyrithione (PT) can efficiently impair the replication of a variety of RNA viruses". Dr. Vladimir Zelenko based his effective protocol after watching the "MedCram Lecture 34 by Dr. Roger Seheult about the use of zinc and zinc ionophores. Zinc is an essential mineral for humans, and a little extra zinc is sometimes used to lessen the intensity of colds and sore throats. An ionophore is a chemical that opens the cell wall to allow minerals (ions) to enter. ‘First do no harm’ Zinc is an over-the-counter supplement. Hydroxychloroquine (HCQ) is a reliable ionophore, and it has a well-established dosing regimen and safety profile." Zinc ionophores "increase the intracellular concentration of Zinc ions causing significant biological effects. Review ionophores: HCQ, EGCG (a plant compound particularly prominent in green tea) and Quercetin (particularly in citrus fruits, apples, onions, parsley, sage, tea, and red wine. Olive oil, grapes, dark cherries, and dark berries such as blueberries, blackberries, and bilberries are also high in quercetin and other flavonoids.) "
Mount Sinai "Copper is a mineral that is found throughout the body. It helps your body make red blood cells and keeps nerve cells and your immune system healthy. It also helps form collagen, a key part of bones and connective tissue. Copper may also act as an antioxidant, reducing free radicals that can damage cells and DNA. Copper helps the body absorb iron. Your body also needs copper to make energy."
Dr. Shiva The Power of Copper to fight virus, bacteria on contact
Copper has even been shown to be very effective at exterminating the much-dreaded hospital ‘superbug’ MRSA. In tests sponsored by the Copper Development Association, a grouping of 100 million MSRA bacteria atrophied and died in a just 90 minutes, when placed on a copper surface at room temperature. The same study found that the same number of MSRA bacteria on both steel and aluminium surfaces actually increased over time. On looking at these figures, many scientists have concluded that the installation of copper-based fixtures such as taps, light switches, door handles, door knobs, pull handles, and push plates in areas such as hospitals could save thousands of lives each year.
Professor Bill Keevil, head of the microbiology group at Southampton University, added his voice to the growing scientific consensus behind this by advocating the use of Copper and Brass door furniture, fixtures and fittings in public places and on public transport, as they could dramatically cut the threat posed by superbugs. In research published in the journal Molecular Genetics of Bacteria Professor Keevil wrote: “There are a lot of bugs on our hands that we are spreading around by touching surfaces. In a public building or mass transport, surfaces cannot be cleaned for long periods of time… Until relatively recently brass was a relatively commonly used surface. On stainless steel surfaces these bacteria can survive for weeks, but on copper surfaces they die within minutes… We live in this new world of stainless steel and plastic, but perhaps we should go back to using brass more instead.” In addition to direct contact killing of bacteria and harmful microbes, amazingly Copper surfaces have been found to exude an antimicrobial 'halo' effect on surrounding non-copper surfaces. Research in the intensive care unit a Hospital in Greece found that other surfaces up to 50 centimetres from copper surfaces experienced 70% microbial reduction, compared to the same surfaces with no proximity to copper-based materials. The ‘Halo’ effect was also observed in trials at a U.S. clinic in 2010. This amazing effect demonstrates just how powerful copper is as a weapon against bacteria." www.morehandles.co.uk
The 1974 Research Act was created in entirety from the Belmont report, and put into place to prevent the Government, it agencies or representatives, military and private companies, from violating an individual's freedom: by forcing, tricking or coercing persons for research, testing and administration of unknown injections/materials, and experimental procedures. This law was enacted after a century long track record of precedence including, and not limited to, the following:
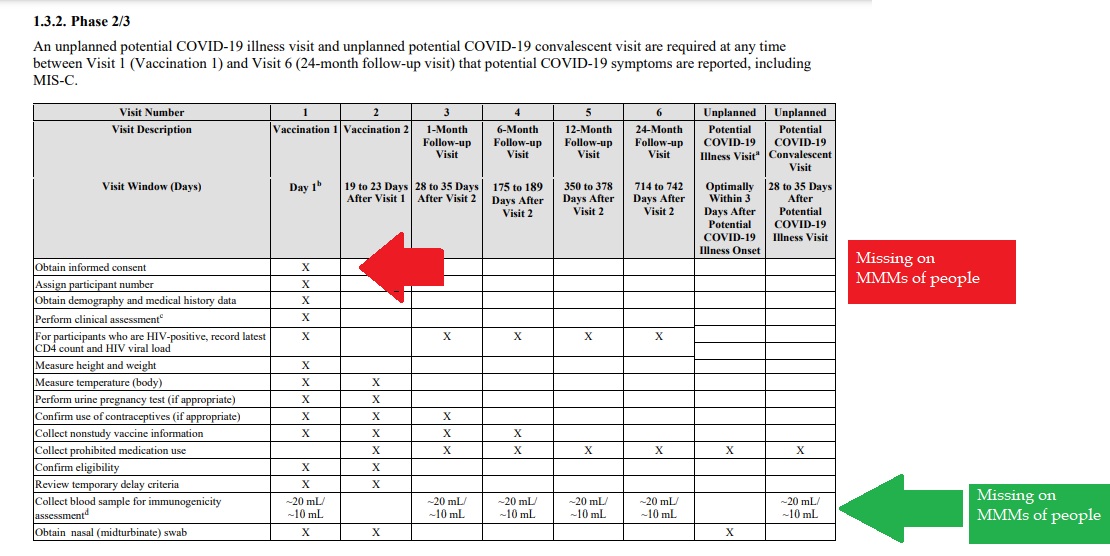
Demonstrated lack of Respect for Persons and their protections in violation of 1974 law. 1- Autonomous agents, individuals capable of deliberation about personal goals and of acting under the direction of such deliberation. The Government and companies have to give weight to autonomous persons' considered opinions and choices, and cannot obstruct their actions and judgments, nor deny individual freedom to act on considered judgments, and cannot withhold information necessary to make a considered judgment. Necessary information includes - but not limited to - all medical opinions by established, industry experts, health status, the necessary assessments, labs, with close monitoring of physical lab and test follow ups of each and every autonomous persons as part of clinical research and testing, along with full documentation of testing, efficacy, use of chimera for research and testing, any and all conceivable side effects, and interactions of conditions. 2- persons with diminished autonomy are entitled to added protections. Violators to the law have been marketing to the most vulnerable, including the immature and the incapacitated who were in need of extra, added protections, even to the point of excluding them from any injections or procedures which may harm them; violating added safety precautions for children, elderly, or those with diminished capacity. Informed consent - must include full disclosure of ALL contents to be injected, any and ALL possible side effects (which can be several pages long), how those persons are individually to be closely monitored, safety guidelines, and above all the right to say no before or at any time, and full reporting of all individuals regarding their safety monitoring/labs/assessments, and any and all side effects. By promoting COVID shots all still under clinical trial/research, and coercing with careers/jobs, inability to travel, etc. in order to take the shots, this law is being violated through: lack of informed consent, lack of protections of autonomous persons, and/or illegally acting as IRB safety board members marketing to those not autonomous and capable of self-determination, with higher standards of protection to be invoked, and assuming the role for their safety. The maxim "do no harm" has long been a fundamental principle of medical ethics. Claude Bernard extended it to the realm of research, saying that one should not injure one person regardless of the benefits that might come to others. An agreement to participate in research constitutes a valid consent only if voluntarily given. This element of informed consent requires conditions free of coercion and undue influence. Undue influence also includes offers of an excessive, unwarranted, inappropriate or improper reward or other overture in order to obtain compliance. Also, inducements that would ordinarily be acceptable may become undue influences if the subject is especially vulnerable as in the case of targeting children, persons with limited capacity, and elderly with elements of mental defect, or instilling fear. Short term morbidity and mortality cases from the shots are well reported and known, such as death, myocarditis along with spontaneous cardiac arrest with no warning, debilitating neurological conditions, etc. And, there is no means yet to determine mid and long term effects because Phase I trials have not been competed, let alone Phase II and III - which is vital information in order to determine informed consent. Injustice has been performed with companies and government representatives, by involving vulnerable subjects, including the young, those unable to fully comprehend with all necessary information, and scaring parents with compromised capacity for free consent. In addition to lack of individual, tightly scheduled, continual monitoring and follow ups, autopsies of all persons involved in this trial participation should be conducted for reporting by the pharmaceuticals companies for any and all persons who received the shots, as well as labs determining efficacy and detriments (as examples, antigen creation, D-dimer, Pulse Cardiac and Troponin Tests) for all those who were coerced or unduly influenced to participate in research. This law was created to protect people from government abuse through experimentation. The government cannot arbitrarily dismiss components, create resolutions or stipulations to supersede the law, as to invalidate its protection of individuals from them, including, but not limited to Health and Human Services (HHS) Center for Disease Control (CDC), Food and Drug Administration (FDA), National Institute for Health (NIH), etc. and pharmaceutical companies, etc. Persons have been illegally acting as members of, or bypassing, IRB safety review and monitoring of each and every person receiving injections, with open undue influence and coercion, to participate in Covid injections. Coercion has been especially directed to the diminished autonomous, children and elderly, through TV ads, library recordings, verbal encouragement, schools or other public venues acting as government agents, and/or clinical researcher recruiting participants, and/or illegally as untrained IRB member who is not following up to ensure safety of the people they recruited, coerced or used undue influence. Overall lack of informed consent has become too often common practice across the healthcare industry, including people being given consent forms hours or minutes prior to surgery; no alternative treatments or lifestyle-nutrition changes prior to medications begin prescribed, and undue influence to intubate or perform surgery on patients in lieu of alternative treatments.
Lawsuits for unconstitutional violation of laws regarding shotsPosted by Brian Ward on Twitter Guess what? Defendants in our lawsuits no longer argue that they had a right to mandate EUA drugs due to their state's at-will employment doctrine. That the EUA drugs can be mandated. That they had the authority to even issue the mandate. Why? Read the 127 pages, and then you'll understand that it was a legal lie from the beginning, but due to the novelty of the laws, no one knew of them. As courts have stated, "sometimes laws take naps," and these laws never showed up for work until now. https://coloradomedicalfreedom.com/wp-content/uploads/2023/08/Stamped-Final-Complaint.pdf full document https://coloradomedicalfreedom.com/wp-content/uploads/2023/08/Stamped-Final-Complaint.pdf page 127 C. the Secretary has no “authority to require any person to carry out any activity that becomes lawful pursuant to an authorization under this section…” 549. In 2005 Congress passed the PREP Act94 which provided the following regarding preemption of state law: (8) During the effective period of a declaration under subsection (b)…no State or political subdivision of a State may establish, enforce, or continue in effect with respect to a covered countermeasure any provision of law or legal requirement that— (A) is different from, or is in conflict with, any requirement applicable under this section; and (B) relates to the…administration…of the covered countermeasure, or to any matter included in a requirement applicable to the covered countermeasure under this section or any other provision of this chapter, or under the Federal Food, Drug, and Cosmetic Act [21 U.S.C. 301 et seq.]. 550. Therefore, via the PREP Act and 21 U.S.C. §360bbb-3, Congress expressly prohibits Defendants from: A. interfering with the authority of the Secretary, B. establishing a condition not authorized by the Secretary, C. establish conditions contrary to the Secretary and the congressional statute under 21 U.S.C. §360bbb-3, D. mandate participation in any 21 U.S.C. §360bbb-3 product or PREP Act activity, E. interfere with an individual considering participation in a 21 U.S.C. §360bbb-3 product or PREP Act activity, F. penalize a person refusing to participate in a PREP Act product or activity or 21 U.S.C. §360bbb-3 product. 551. The executive branch of the United States Government purchased all COVID-19 licensed and EUA drugs using federal funds. Congress expressly prohibits the federal government Brian Ward @GodsRiddles Nov 1, 2023 Breaking…Gov Gavin Newsom and Kaiser have been sued in federal court for requiring healthcare workers to inject an experimental drug into their bodies as a condition to sell their labors in the marketplace. The requirement violated the workers’ Equal Protection of Laws and Due Process rights. Moreover, Kaiser signed a contract with the CDC promising not to mandate participation but did so anyway. Kaiser and Newsom fraudulently concealed the fact that nurses would be required to forfeit litigation rights if they incurred an injury from the use of the experimental drug. The California Nurses union stood by and refused to stop Newsom’s tyranny, leaving members without representation. Governor Newsom perpetuated the greatest assault on the US Constitution in the state’s history and the CA AG did nothing to prevent it. Worse yet, is that CA and Kaiser already had an agreement with HHS promising to never place an individual under a sanction for refusing to inject federally funded experimental drugs into the body. Legal Fact: medical providers have dual roles in relation to their employees. An employee can be a patient and an employee. Should a hospital mandate the use of a drug under the PREP Act as a condition of employment and the patient is injured then the patient would find it difficult to sue the medical provider for that injury. However, the employee has the right to seek compensation irrespective of the PREP Act because it’s an on the job injury. Of course this opinion does not account for the laws of all 50 states but demonstrates why one should seek legal advice immediately upon sustaining an injury. This is not my opinion but the chief judge of the 11th circuit who wrote a slip opinion when he was AL AG denoting the company is liable for vaccine injuries because the company believes the vaccine would benefit the company. Last edited10:55 AM · Nov 10, 2023
Brian Ward - Twitter Oct 25, 2023 Legal Fact: Any state that penalized citizens or denied unemployment benefits to an individual terminated for the sole reason of refusing to inject an unlicensed investigational drug into their body violated the individual's Fourteenth Amendment Equal Protection and Due Process rights. The U.S. Congress conferred legal authority onto an individual to either accept or refuse an EUA product. Both options were enacted by a valid act of Congress and must be equally protected. Moreover, the Supremacy Clause of the Constitution denied that state authority from interfering in the chosen option. The state established what courts call an Unconstitutional Condition. SCOTUS said: "But the power of the state in that respect is not unlimited; and one of the limitations is that it may not impose conditions which require the relinquishment of constitutional rights. If the state may compel the surrender of one constitutional right as a condition of its favor, it may, in like manner, compel a surrender of all. It is inconceivable that guaranties embedded in the Constitution of the United States may thus be manipulated out of existence." Governors and state agency directors violated their oath of office & the Constitution the moment they required COVID-19 EUA participation and or penalized an individual's federally protected option. They deprived citizens of their interest in liberty and property and, unfortunately for some, their very lives, all without due process. They did so by applying the law unequally demoting those who refused to that of a second-class citizen. Legal Fact: 21 U.S.C. §360bbb-3 and the PREP Act expressly restrict public and private employers from conditioning access to employment and other benefits upon a person injecting an FDA-classified experimental drug (Pfizer-BioNTech COVID-19 Vaccine) into their body. The CDC stated, "Coverage under the Public Readiness and Emergency Preparedness (PREP) Act extends to Organization if it complies with the PREP Act and the PREP Act Declaration of the Secretary of Health and Human Services." "IF IT COMPLIES" is the key phrase here. When a governor issued a proclamation that directly violated the federal statute, the governor did not comply and fraudulently amended the federal law in violation of the Supremacy Clause. The PREP Act and 21 U.S.C. §360bbb-3 provide a legal right to individuals considering participation in the product/activity. That legal right is the option to accept or refuse. Therefore, the immunities provided to persons participating in activities under the PREP Act extend only so far as they do not force persons to participate in the product/activity. Governors, employers, hospitals, all FORCED (under threat of a penalty) individuals to participate in violation of federal law. Force does not have to mean physical force. It can be the force of law, rule, or other means having the same negative effect as physical force. The courts have said that force means to prevent a person or cause a person to participate in an activity outside of their free will and voluntary consent. Significant lawsuits inbound! 12:45 PM · Aug 29, 2023 Brian Ward Aug 2022 BREAKING: LA Superior Court requiring LAPD to reinstate an officer terminated for refusing the vax mandate. Full back pay too! This is under a Writ of Mandamus, which is rare. Legal Fact: "Nothing in this section (EUA Law) provides the [HHS ] Secretary any authority to require any person to carry out any activity that becomes lawful pursuant to an authorization under this section, and no person is required to inform the Secretary that the person will not be carrying out such activity." - The Secretary may grant access to an unlicensed drug (Pfizer-BioNTech COVID-19 Vaccine) during an emergency but he can not mandate that anyone manufacture, distribute, store, administer, or receive the product. His authority is non-transferable, nor may he delegate it to another person. Therefore, by what authority are universities and private employers mandating that which Congress prohibits? EUA drugs are "controlled" drugs by Congress, and no person may participate in them outside of the conditions established by Congress. Moreover, Congress expressly prohibits private employers from interfering with your choice of accepting or refusing participation in the product. It was illegal - It is illegal - and it is being remedied in court. We will not allow this September to be a repeat of years past. 2:18 PM · Aug 21, 2023 Legal Fact: 100% of all hospitals and nearly all universities signed a FEDERAL agreement to abide by the ethical principles of the Belmont Report, though few know it, ANYTIME they involve a human with an investigational medical product such as any available COVID-19 drug. The report is only 10 pages long, but it holds in part: (1) Respect for persons incorporates at least two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection. The principle of respect for persons thus divides into two separate moral requirements: the requirement to acknowledge autonomy and the requirement to protect those with diminished autonomy, (2) To show lack of respect for an autonomous agent is to repudiate that person's considered judgments, to deny an individual the freedom to act on those considered judgments, or to withhold information necessary to make a considered judgment, (3) Respect for persons requires that subjects, to the degree that they are capable, be given the opportunity to choose what shall or shall not happen to them. BONUS ROUND - All US States and Territories signed this agreement pre-pandemic too. Yup, no one knows this either, including state attorneys general. Lastly, unlike the Nuremberg Code, the Belmont Report has the force of law via federal statute and contract. Last edited9:28 PM · Aug 2, 2023 Brian Ward via Twitter
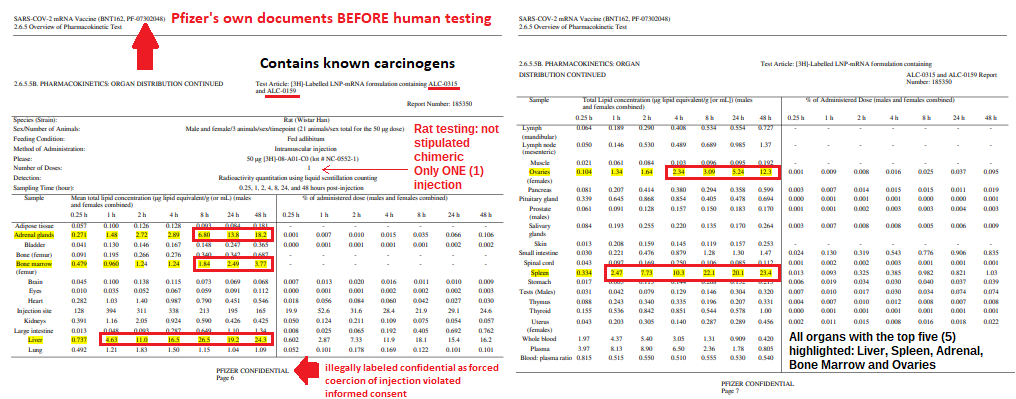
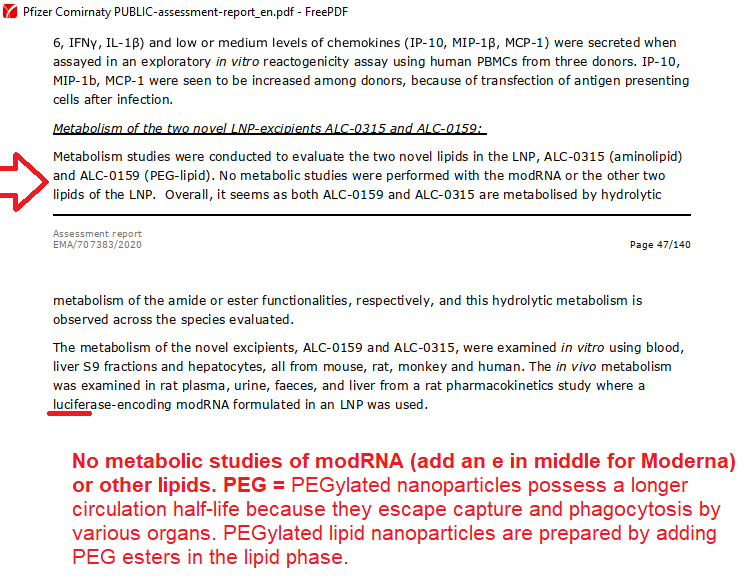
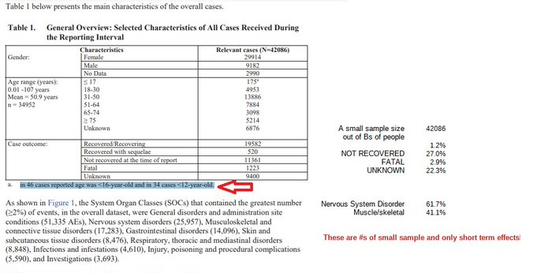
The VERY first EUA issued was in 2005 for the Anthrax investigational drug for service members and civilian employees of the DoD. The EUA stated: A. Individuals (service members and civilians) who refuse anthrax vaccination will not be punished. (Emphasis added) B. Refusal may not be grounds for any disciplinary action under the Uniform Code of Military Justice. C. Refusal may not be grounds for any adverse personnel action. Nor would either military or civilian personnel be considered non-deployable or processed for separation based on refusal of anthrax vaccination. D. There may be no penalty or loss of entitlement for refusing anthrax vaccination, E. This information shall read in the trifold brochure provided to potential vaccine recipients as follows: You may refuse anthrax vaccination under the EUA, and you will not be punished. No disciplinary action or adverse personnel action will be taken. You will not be processed for separation, and you will still be deployable. There will be no penalty or loss of entitlement for refusing anthrax vaccination. Nothing in law has changed to negate the authority of DoD members to refuse EUA COVID-19 drugs except the 6 civilian appointees engaged in willful misconduct against our Armed Forces. 11:51 PM · Sep 3, 2023 by Rose M. RohloffProviding informative snippets from 100,000s of primary source documents. None of this gain-of-function (GOF) work meets the legal definition of a vaccine. Link exhibit (A) with exhibit (C) regarding active substance; injections with GOF. Chimera used for creation, efficacy testing (human+animal); Humanized mice from injected aborted fetus cells, to create LUCIFERase encoded modRNA: active substance is SPIKE PROTEIN.
World Council For Health - Detox Protocol https://worldcouncilforhealth.org/resources/spike-protein-detox-guide/ Flu shots
read blog entry 9/24/23
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
by Rose RohloffWrite something about yourself. No need to be fancy, just an overview. The current fulminated issues of "hospital kidnapping", and denying Rights of parents has existed for decades. Now health systems are using Department of Children Family Services (DCFS) to enforce. The following are true, documented cases of families, spanning decades, fighting hospitals for the health of their children. |
| Other parents, and children of elderly parents, across the country are experiencing similar situations of coercion, threats and intimidation from hospital administrators - including calling DCFS and the police; forcing families to hire expensive lawyers to even get a basic second opinion from a clinician they trust, and the stress with fear of actually having to go to court to fight for their loved one's Rights, who are being held (kidnapped) |
| One example of a form. A signed form should include the responsibility of hospital, staff enforcing quality care standards (e.g. to avoid hospital acquired infections and conditions), ensured care planning and care coordination, etc. Blanket consent is not informed consent, and should not include coercion upon ER visit with emotional compromised states. Being a signed form, a copy should always be immediately given to the person signing. |
| There are many classifications of drugs used for cardiac and vascular (CV) issues. A 2007 report is cited below, with his overviews by drug classification. The full article can be read with this link. |
Neuropsychiatric Consequences of Cardiovascular Medications
by Dr. Jeff C. Huffman
Associate Professor of Psychiatry at Harvard Medical School and the Director of the Cardiac Psychiatry Research Program in the Massachusetts General Hospital (MGH) Division of Psychiatry and Medicine.
Angiotensin-converting enzyme inhibitors
https://pharmaceutical-journal.com/article/news/from-snake-venom-to-ace-inhibitor-the-discovery-and-rise-of-captopril
Beta-adrenergic blocking agents or Beta (β)-Blockers
Calcium channel blockers (CCBs)
Diuretics
Doctors should always monitor electrolyte levels (sodium/Na and potassium/K+) of their patients taking diuretics.
Centrally acting agents - Antiadrenergic agent
Bottom line: Methyldopa is clearly associated with fatigue and sedation. In contrast to early studies linking methyldopa with depression, later reviews and studies have found this association to be relatively weak. Other neuropsychiatrie symptoms are uncommon.
Bottom line: Reserpine is associated with both sedation and daytime fatigue. Incidence of depression may be elevated among patients taking reserpine. However, other (generally more recent) reports question this association."
α-Adrenergic agents
Vasodialtors
Antiarrhythmic medications
Bottom line: Class III Amiodarone is associated with thyroid abnormalities in 15% of patients, and untreated thyroid dysregulation can lead to a variety of mood, cognitive, and psychotic symptoms. In contrast, direct neuropsychiatrie effects of amiodarone are uncommon.
Bottom line: Digoxin is associated with delirium and other cognitive effects, especially in toxicity. Visual changes and hallucinations may also occur with digoxin use, even at normal serum levels."
Conclusion by Author
Patients and their families know the cognitive baseline of individuals being prescribed medications, and therefore, should always monitor for any neuropsych impact seen if CV drugs are used, on an individual basis for what is safe per person. Any and all side effects should be know, along with contradictions to other drugs, for all medication being taken.
What should you be aware of regarding Vancomycin?
| Vancomycin (Vanco) is an antibiotic, in the classification of Glycopeptide used for gram (+) bacteria, typically used for: Methicillin Resistant Staphylococcus Aureus (MRSA); Clostridium Difficile (commonly called C-diff), a potentially deadly infectious |
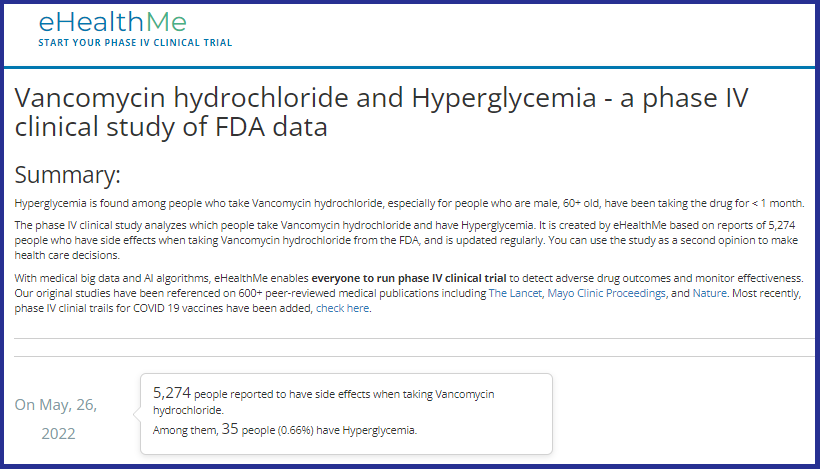
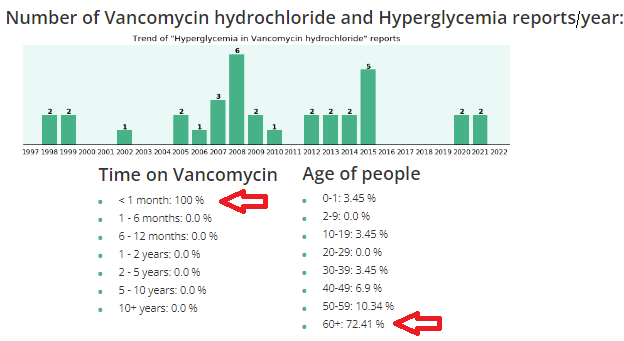
Clinicians (nurses, doctors, physician assistants, etc.) are supposed to do comprehensive history and physicals (H&Ps) before using any drugs or treatments. And, it is important to know underlying conditions before using Vanco, because it can raise glucose levels, especially in diabetics, and/or cause kidney (renal) insufficiency, especially in elderly.
"I don't know what the average "lay person" does when they don't have all this information, and without a medical person to look out for them."
"This article is exactly why they stopped Vanco when he had his MRSA infection. His kidneys were starting to get compromised, so they immediately stopped it and started him on a different antibiotic, Daptomycin, which worked just as well and had less side effects for his kidneys and diabetes. Thankfully they caught it very early because of the blood tests they were doing to see what was happening. The new antibiotic was infused only once a day as opposed to twice a day Vanco, and there weren't all the extra blood draws to make sure the kidneys and glucose were doing okay. God is really in control and watching out for us, because the medical field doesn't always do that, even with a fellow medical person."
Vanco and high glucose/hyperglycemia
Vanco and kidney (renal) failure
Abstract A progressive increase in vancomycin resistance with consequent treatment failure has been observed in staphylococci. Therefore, new dosing guidelines advocating much higher vancomycin doses have been issued. Target trough levels of 15-20 microg/ml are proposed. Whether and how these targets can be achieved in patients with chronic kidney disease or those on dialysis are still under evaluation. The higher vancomycin doses to achieve these treatment targets carry a substantial risk for nephrotoxicity. This risk is incremental with higher trough levels and longer duration of vancomycin use. Critically ill patients, patients receiving concomitant nephrotoxic agents, and patients with already compromised renal function are particularly at risk for vancomycin-induced nephrotoxicity.
Acute kidney injury during daptomycin versus vancomycin treatment in cardiovascular critically ill
by Rose Rohloff
Championing care: knowing your individual baselines, understanding trending deviations, influencing factors.
| A Wall Street Journal (WSJ) article https://lnkd.in/e9awgQT 1/24/2020 posted wrote: "Nearly 150 years ago, a German physician analyzed a million temperatures from 25,000 patients and concluded that normal human body temperature is 98.6 degrees Fahrenheit. That standard has been published in numerous medical texts and helped generations of parents judge the gravity of a child’s illness. But at least two dozen modern studies have concluded the number is too high. Or was it? In a new study, researchers from Stanford University argue that Wunderlich’s number was correct at the time but is no longer accurate because the human body has changed. Today, they say, the average normal human-body temperature is closer to 97.5 degrees Fahrenheit." | Championing your own health is about understanding your healthy baselines. Just as individuals have a variable "healthy" baseline for their blood pressure and heart rate, why would an individual not have an individualized, normal baseline for their temperature? And, temperatures are now measured using different methods: forehead-ear-mouth-rectal, producing deviations based upon how the temperature is obtained. It is important to understand, compare what mechanism was used to take the temperature. Is your normal 98 degrees, while another may run 97 degrees? Is the person normally running 97 degrees now have a temp of 99 along with malaise, dehydration because they are fighting a virus or bacteria? Assessment/vital sign numbers provide more information for clinicians (Doctor, PA, Nurse, etc.) when provided in context of associations, trends, baselines and influencing factors. |
by Rose Rohloff
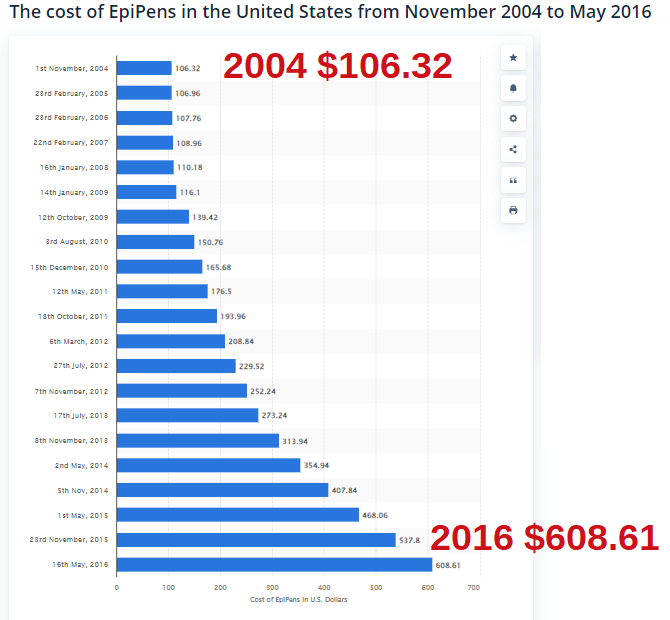
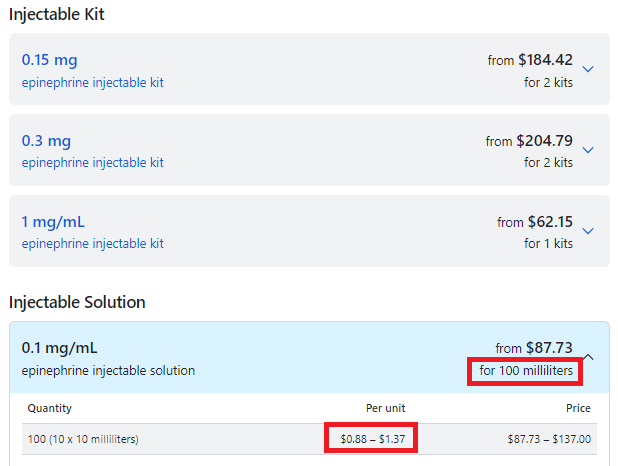
| This needs to be addressed at the State & Federal levels - fighting the unconstitutional Federal violation of the Sherman Antitrust Act of 1890 by Congress and the Executive Branch. [The Federal Gov is also violating pushing for Narcan to be in every household - pouring gasoline on fires - because now people think they can be saved continuing to overdose on street drugs, especially Fentanyl: read NARC PARTY - OPIOID CRISIS AND SURGEON GENERAL ADVISORY | How Mylan, the maker of EpiPen, became a virtual monopoly - The Washington Post washingtonpost.com› business › economy › 2016 › 08 › 25 › 7f83728a-6aee-11e6-ba32-5a4bf5aad4fa_story.html August 26, 2016 - The company lobbied lawmakers — both directly and indirectly — to increase the availability of epinephrine autoinjectors in U.S. schools. |
Use Case: A police officer was having hives and went to an emergency room. The staff asked him if he wanted an EpiPen. His response, "What the hell is it? The staff did not educated him, provide one to him to use if he felt he needed it. I informed him to read the fine print = it was a dose for a child an not a grown 200lb man, with an expiring date in a couple of weeks.
| On November 13, 2013, President Obama signed into law the School Access to Emergency Epinephrine Act. The federal legislation provides a financial incentive for states to enact their own laws requiring schools to keep non-student specific epinephrine auto-injectors in case of an emergency. And so, the Federal Government created an illegal monopoly for company who owns the patented auto-injector, not the medication, followed by price gouging, especially for times of crisis, and finally panicked outrage in the public. |
|
| 'There's nothing to give them': Patients, pharmacists scrounging for EpiPens Full story Patients and pharmacists nationwide are grappling with a persistent shortage of Mylan's EpiPen, forcing some to travel great distances or go through several hoops to access the lifesaving allergy treatment, according to Bloomberg. EpiPens have been difficult to obtain since at least May 2018, when the FDA alerted the public to the shortage. |
| Cheaper "do-it-yourself" alternative EpiPen may carry more ...https://www.cbsnews.com/news/cheaper-epipen-alternative-may-carry-risks/ The cost is about $20 - $15 for a syringe and $5 for a vial or two of the drug. |
Summary
- Obama signed into law an illegal monopoly
- The Mylan company does not control the patent on the drug epinephrine, only their autoinjector Epipen.
- Epinephrine comes in multi-does vials: EpiPens are only single dose!
- CDC stats read 4%-6% have food allergies, only .001 of the population has anaphylaxis to nuts + shell fish, the biggest allergy issues, 63 to 99 deaths occur each year in the United States due to anaphylaxis.
Kyle Reyes, CEO, The Silent Partner Marketing
The solution is bringing analysis down to the most base level of management with front line analysis, to coincide with first-hand observation, the voice of the patient & their caregivers/champions, and reducing the ever growing administrative overhead. Bigger is not better for addressing health and care of populations, when the focus is shifted upward with large systems where individuals are lost: Especially when the individual issues are indicative of the core problems that need to be addressed for quality care delivery.
The need for P&P Reviews
by Rose Rohloff
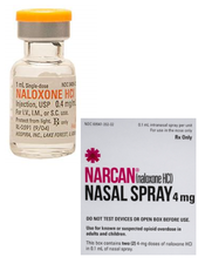
This week, reports were released in the media that US homes need Narcan to aid in opioid overdose epidemic, surgeon general advises
The general public needs to be aware: Naloxone has been reported to foster increased abuse of drugs by allowing revival of overdosing for continuing to take more drugs. Naloxone is the generic of Narcan. Just as EpiPen is only the delivery system and not the generic drug Epinephrine, It is important to know the difference between the brand name versus the generic drug name.
The danger of advising the untrained public to distribute emergency medicine
Many clinicians, let alone the general public, are not specifically trained in the proper dosage and treatment with Naloxone for the various forms and dosages of opioids and heroin.
FDA Advisory Committee on the Most Appropriate Dose or Doses of Naloxone to Reverse the Effects of Life-threatening Opioid Overdose ... Sept 2016
"The effectiveness of naloxone, and thus the exposure required, will depend on the opioid dose, the potency of the opioid in binding receptors, the lipophilicity of the opioid in crossing into the CNS system and the elimination half-life of the opioid, together with patient factors (7, 26). Appendix [2] and [2a] includes further information on naloxone pharmacology. The complex pharmacology of appropriate dosing is further compounded as often the fentanyl involved is illicitly manufactured without normal procedures or controls and may be introduced surreptitiously into heroin or prescription painkillers. Reports from the field confirm the need for additional naloxone doses to reverse opioid overdoses including those involving more potent fast onset synthetic opioids."
Narcan (Naloxone HCL) Use in Opiod Overdose: A Perspective
by Joan M. Rider-Becker, BS, PharmD, FMPA
Retired, Emeritus Professor, Pharmacy Practice Ferris State University College of Pharmacy Education/Training
B.S. Pharmacy-Ferris State University College of Pharmacy-1987
Pharmacy Practice Residency-Bronson Hospital Kalamazoo, MI-1987-1988
Doctor of Pharmacy (PharmD), University of Michigan-College of Pharmacy Ann Arbor, MI 1990
I have used opioids now for chronic pain management after a car accident almost twenty-years ago. I will admit, I was taken aback by my family physician about a month ago being given a prescription for Narcan (generic name Naloxone) as a “precautionary measure” for my chronic opioid use. The form I was prescribed is a nasal formulation vs. the oral/injection form. When I took it to a pharmacy to be filled, I had to undergo “special counseling” by a pharmacist (even with my credentials) which consisted of a video on proper use and a warning that after use, 911 had to be called and I was to be taken to the emergency room for follow-up. This is the proper follow-up when someone is prescribed any rescue medication for a drug reaction. The Naloxone is only to be given when a known opioid (i.e. codeine and it’s derivatives; Fentanyl, Meperidine, etc...) is given or taken in life-threatening incidences. I was instructed, "Were you aware that Naloxone has two elimination half-lives because this drug has an active metabolite; and, were you aware that Naloxone and Naltrexone are different agents, but are easily confused."
I believe giving someone this agent for overdose situations is giving a false sense of security that nothing else needs to be done. Nasal Naloxone is like putting a bandage on a cut artery. You may stop the blood flow at the moment, but the wound will continue to bleed if the wound isn’t sutured properly. Without appropriate emergency room follow up of an opioid overdose the person may die from that overdose.
Many opioids vary in dose, strength, predictability and most of all drug half-life. Knowing the half-life of drugs is essential to know how long the drug is going to last in your body. Drug half-life’s, drug absorption, distribution and elimination is well covered in Colleges of Pharmacy in courses such as pharmacology, pharmacokinetics and pharmacotherapeutics. Pharmacists do not know the pharmacokinetics on every drug substance out there by memory, and we are called the drug experts. Physicians do not have nearly as much education on medications as pharmacists, yet they are the first line of treating drug overdoses in emergency situations along with the nurses, Physician Assistants and Nurse Practitioners.
The general public is being provided a false sense of security by the media to carry this drug in their homes to address the opioid crisis. The public needs to be AWARE there is more to treating an opioid overdose than just squirting this agent up their nose.
by Rose M. RohloffMany articles and discussions have centered around the rise and demise of Theranos. The company valuation and strategy were based on their technology for running multiple tests, at reduced cost, utilizing a pin-prick instead of a regular needle blood draw. Learned lessons from the devaluation and closing of the labs and blood testing centers include the lack of transparency, the need for thoroughly vetting new innovation, the requirement to understand the actual market, the need to support vision with qualified proof-of-concept, as well as deficient oversight and due diligence - to name a few. One lesson of success, however, has been overlooked: The model of a needed paradigm was beginning to be established. Leaders in the healthcare industry tout the need for enabling consumers to be more engaged with their own health. The vision for Theranos to offer new blood testing technology also precipitated three (3) key factors supporting increased consumerism: | 1- having lab results sent simultaneously to consumers/customers at the same time to their physicians, within 24 hours; 2- the passage of AZ law HB 2645, enabling individuals to order their own blood tests from a licensed clinical lab without a doctor's order, paying cash; and 3- easier access to get lab tests performed through multiple, local health and wellness pharmacies, instead of going to a lab or hospital. This paradigm has not been discussed as successful, and one key component for involvement with control over one's own health, expanding the traditional care continuum to a health maintenance continuum. |
With the many lessons learned from an aborted (shady) startup, marketed to disrupt the healthcare industry with its counterfeit technology, the direction for having consumer-driven as the process should not be overlooked - and actually be revisited to expand upon the great health ownership model of good disruption to healthcare.
Ms. Rohloff, a 35-year healthcare veteran with experience in nursing, business and information systems, spoke with Becker's Hospital Review about providing consumers with more detailed evaluation of quality care delivery. [read more]
click an article
to read and post comments
Search topic
select category
All
APAC Teams
Care Quality
Champion Your Own Care
Clinician Quality Education
Experience Satisfaction
Healthcare Consumerism
Health Innovation
Medical Care Coordination
Palliative Care
Patient Engagement
Physicians
Population Health
Transitioning Care Coverage
search by date
July 2024
June 2024
April 2024
March 2024
February 2024
January 2024
November 2023
October 2023
June 2023
May 2023
October 2022
September 2022
July 2022
August 2020
April 2020
January 2020
June 2019
May 2019
April 2019
October 2018
September 2018
August 2018
July 2018
May 2018
April 2018
March 2018
February 2018
January 2018
December 2017
September 2017
June 2017
October 2016
September 2016
May 2016
March 2016
October 2015
June 2015